Fail-safe neutralization of wastewater effluents
An overview of equipment, systems,
and materials
Gary Kaminski, Systems Engineer, Vanton Pump & Equipment Corp.
Hillside, New Jersey
There was a time when industrial facilities paid little or no attention
to the waste streams from their plumbing systems. Whatever went down the
sinks, toilets, or laboratory and plant system drains went into the sewers
or open ponds on their property. Sometimes these wastes were piped into
running streams that ran through or bordered on plant property. Out of
sight, out of mind was the order of the day.
But that's all behind us now. In fact, the beginning of the end began
in 1970 when the Environmental Protection Agency was established. This
was soon followed with a national permit program requiring specific deadlines
for the control of industrial plant discharges into the atmosphere as well
as into the nation's water system.
As is the case with most political activity, regardless of intent, there
followed a series of laws, regulations, and interpretations. These new
constraints led to the creation and proliferation of legal specialists
and compliance consultants to help plant managers understand the laws and
avoid financial and corporate image penalties. This article deals with
only one segment of this responsibility--the treatment of industrial wastewater
to satisfy the neutralization requirements of the National Pollutant Discharge
Elimination System (NPDES) and other regulations that require that fluid
effluents contaminated with acids or alkalis be neutralized before being
discharged into the public sewer system.
The argument that the acidic or alkaline contribution to multi-billion
gallon municipal water systems by an individual industrial firm would be
minuscule lost its validity when these small additions were considered
collectively. Facts and figures proved rather conclusively that the combined
contamination complicated controls and processing operations of the municipality,
making it almost impossible for it to meet the mandated federal neutralization
requirements.
Since penalties for failure are substantial, from $5,000 to $50,000
and more per day, the onus is on our backs. It is extremely important for
plant
management to understand what it takes to achieve acceptable pH neutralization
and the most cost-effective means for assuring fail-safe operation of the
neutralization system.
Liquid waste management problems are as diverse as the industries that
produce them. Whatever your plant, chances are you are vulnerable. As long
as your company uses acids or caustics, the law requires you to neutralize
the effluent so it conforms to local as well as federal pH requirements.
The ultimate aim of environmental regulations is to achieve the impossible:
zero discharge of industrial pollutants. A more realistic approach, however,
is one that requires you to meet acceptable waste standards established
by the local communities. This implies that local plant managers can play
an important role in determining what these standards should be in light
of their plant's ability to conform, in negotiating variations from the
requirements where appropriate, or in securing meaningful time-to-conform
periods.
Understanding the pH requirement
Just what does it mean when the regulation requires liquid effluents
to be neutral. The pH is related to the hydrogen-ion concentration
in a liquid. For a solution to be truly neutral, it must be neither acidic
nor alkaline. This is determined by a pH with a numerical value of 7, the
value given to uncontaminated water. The neutralization process is the
chemical reaction of an acid with a caustic substance that results in the
formation of a salt or water. In an aqueous solution, the acid or caustic
molecules disassociate and form ions. For example, with sulfuric acid (H2SO4)
and caustic soda (NaOH), sulfuric acid is present as H+ and
SO4-ions and caustic soda as Na+ OH-
ions. The positive H+ ions of the acid and the negative OH-
ions of the caustic have a strong attraction for each other and combine
to form HOH = H2O = water. This chemical process, the combination
of hydrogen (H) and hydroxide (OH) to form H2O, is referred
to as neutralization. For complete neutralization, there must be a hydrogen
ion for each hydroxide ion. If there is surplus of either, the solution
is acid or alkaline, with a pH below 7 (acid) or above 7 (alkaline).
The significance of one number up or down is seen in the fact that the
pH scale is logarithmic. Each number expresses a 10-times higher or lower
concentration of the acid or alkali. The wider the acceptable range, the
less costly is the system you need to install and the instrumentation it
requires. When designing or installing a neutralizing system, it pays dividends
not only to know the
existing regulations but the trend and the potential for change in
your own
municipal district.
Neutralization systems can be either batch or continuous. For the great
majority of industrial plants batch neutralization systems are the most
cost-effective. Since these tend to be cyclical in nature, with volume
and characteristics uniform, instrumentation is not as complex as it would
be for a continuous system.
Fail-safe systems generally involve a predetermined tank size for the
gathering of the waste fluids, dosing or metering the required acid
or caustic as determined by pH sensors, mixing of the neutralizing chemical
with the waste fluid, controlling the fluid level in the tank, and discharging
the neutralized fluid into the sewer system.
Continuous treatment systems, on the other hand, require more sophisticated
pH sensing, dosing, mixing, and discharging arrangements, including downstream
pH sensing systems and additional equipment and controls for recirculation
and reneutralization if the discharge is not within prescribed limits.
Both systems, batch or continuous, should be equipped with appropriate
audible and visual alarms as well as permanent data-recording instrumentation.
These are needed to ensure the discharge is in compliance and to prove
that compliance to visiting government inspection teams.
Management Involvement is critical in three areas. Separate fact
from fiction. Only you can get the facts on the chemicals you are handling
and the waste fluids your plant is generating.
Explore the possibility of a process or chemical concentration change
that might affect neutralization. Review the potentials for recycling costly
chemicals.
Although this approach is not directly related to fail-safe considerations,
it's a good starting point to reducing your waste neutralization problems
and it helps to understand the margin for error you can tolerate. It directly
affects the level of instrumentation and record-keeping you need to incorporate
in your system. It also helps to pinpoint your potential liability in case
of failure.
Familiarize yourself with the federal, state, and municipal regulations
that affect liquid discharge into the municipal system. Take the time to
meet with your state and municipal officials and don't ignore the town
engineer. The more you know about local capabilities for handling liquid
plant wastes, the better.
Assess the significance of the various potentials for system
failure with respect to the equipment and controls you have or might have
to install. These are basic to the critical management decisions that impact
on fail-safe operations.
System design factors
Equipment selection is dictated by volume requirements, plant space
availability and the nature of the effluents. Consideration must be given
to deciding whether to locate the neutralization facility within the plant
walls or to gather the waste fluids outside and conduct the processing
in an outbuilding or in the open. Of equal significance are the decisions
relating to the choice of construction materials. Should the system be
stainless steel, glass-reinforced thermosets, or thermoplastics? Should
material selection be determined by the specific fluids now being discharged
or with a review of future needs or the potential for unknown or changing
characteristics of the wastes?
We address these concerns by presenting a flow diagram of a typical
neutralization system followed by a discussion of the critical factors
related to equipment and material selection.
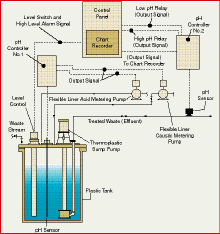 Self-contained
neutralization systems Self-contained
neutralization systems
Figure 1 illustrates a typical neutralization system having a single
pump and relatively simple controls. It's a batch type system in which
the waste from process operations has been collected prior to discharging
into the municipal sewer line. A non-rising rod liquid level control activates
a valve in the feed line closing off or diverting the incoming waste stream
when the liquid volume reaches the prescribed setting. A submersed pH sensor
within the tank relays the vital hydrogen-ion concentration information
to a pH analyzer equipped with two 4 to 20 milliamp output signals and
a set of high and low contacts that activates the appropriate metering
or dosing pump, should the pH be outside the allowable limit.
Many municipalities accept streams that are slightly alkaline, with
a pH value between 7 and 9. If the liquid in the tank falls within this
range, a valve in the discharge line is opened and the sump pump is energized.
Should the pH be below 7--acidic--the metering or dosing pump automatically
supplies a flow of caustic to neutralize the batch. As the pH rises above
7 or above the preset level, the metering or dosing pump automatically
stops, and the sump pump activates. The action is identical for a pH above
the preset upper alkaline level. In this case, the acid pump automatically
provides the neutralizing chemical and the sump pump will be activated
when the waste fluid is in the pH range acceptable for discharge. The system
shown indicates a downstream pH sensor to identify and cut off the treated
waste stream if it fails to fall within the acceptable range.
Figure 1 Automated neutralization
system simplifies wastewater regulation compliance when handling corrosive
fluids.
The neutralizing chemicals used most widely are sodium hydroxide and hydrochloric
or sulfuric acid. In many industrial facilities, used or spent process
baths of these chemicals from metal finishing and plating are incorporated
into the neutralization process. It is important that components of the
neutralizing system in contact with the waste stream or with the neutralizing
fluids be manufactured of materials completely resistant to the fluids
at the anticipated operating temperatures. The system illustrated used
polypropylene for the tank, pumps, valves, and piping. The instrumentation
components are selected for compatibility with the waste stream and the
neutralizing chemicals.
The equipment
Storage tanks. Mechanical engineers can readily recommend the
size and structural support you need to contain the anticipated volume,
the special protection against external pressure needed for in-ground tanks,
and similar factors. Management has to ensure that the materials of construction
are compatible with the waste fluids, that every precaution is taken to
ensure leak-tightness, that double wall construction is used where hazardous
materials are involved, that adequate spill containment area and necessary
equipment are provided for, and that spill-handling procedures are delineated
clearly.
High and low level controls. The primary function of these controls
is to start and stop pumping operations automatically. Cost is related
to sophistication and the need for notifying personnel of impending danger.
In a single or multi-pump system, liquid level controls should activate
audible alarms when fluid levels exceed the safety margin.To avoid the
high cost of pump motor burnout from run dry operation, low level controls
should also be set for automatic pump shut off at a designated fluid height.
The positioning of the switch housings for these controls should be
well above the corrosive or hazardous fluid and sealed in watertight, and
where necessary, fume-tight enclosures. In a duplex pumping system, the
controls can be set to operate the pumps on alternate cycles or to operate
both pumps automatically and simultaneously when incoming flow rises above
the capacity of one pump to reduce the fluid height to safe operating levels.
Sensing and controls. Since pH adjustment is the most critical
operation in the neutralization process, fail-safe operation is dependent
on the pH sensor and the controller that activates the chemical feed pumps.
The sensors selected be of highest quality and completely inert to the
waste fluids. The controller must be able to initiate pump action and prevent
overfeeding of either the acid or caustic pump.
Overfeeding of either chemical results in wide pH swings, unnecessary
and repetitive chemical additions, and inaccurate control. This leads to
discharges with unacceptable pH readings and excessive recirculation if
this is picked up by a downstream pH sensor.
Pumps. Vertical centrifugal sump pumps are the type commonly
recommended for use within the tanks of standard or customized neutralization
pump and tank systems. The responsibility for selecting the specific design
or supplier belongs with your engineering department or consultant, but
there are a few questions that management should be asking.
One pertains to assurances that if plastic pumps are specified, there
should be no potential for metal-to-fluid contact. Ensure that the metal
shaft--usually a stainless steel or a special alloy--is completely sheathed
with a thick sectioned plastic sleeve of a material compatible with the
chemical waste being pumped.
Another consideration is the material from which the impeller is molded.
If the fluid contains abrasive material, even though the tank is specified
in polypropylene or PVC, you may want to insist that the impeller be provided
in PVDF which has superior abrasion resistance. The higher cost of the
fluoropolymer will be repaid many times over by lower maintenance and
less downtime.
Another significant pump design related to the handling of fluids that
contain solids or stringy materials is the positioning of the impeller.
Insist on a vortex, recessed impeller pump head to avoid clogging or binding.
Still another costly application problem worth your concern is the potential
for anticipated or unexpected dry run conditions. If this frequent cause
for pump failure is present, consider the use of full cantilever bearingless
designs.
The addition of caustic or acid on demand is usually handled by nonmetallic
precision metering or dosing pumps. Or if accuracies of 5 percent or greater
are permissible, buy less expensive rotary sealless peristaltic types that
have heavy duty elastomeric flexible liners. Selection should be determined
by the degree of accuracy required and the availability of the most suitable
pump construction material for the chemical being metered.
Materials. Corrosion is the greatest enemy of fail-safe equipment
handling process chemicals and those used for cleaning, pickling, metal
finishing, surface treatment or laboratory operations. In addition, variable
and mixed waste streams present a wide range of material selection problems.
This much we know. All metals corrode, some more rapidly than others.
For this reason, tanks, pumps, fittings, valves, and piping for neutralizing
systems are recommended in nonmetallic materials, as long as temperatures
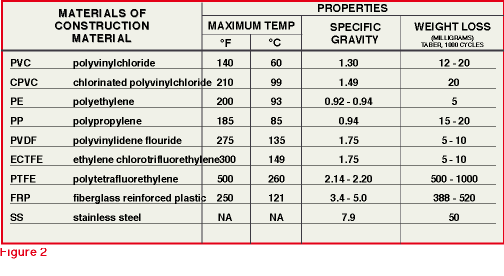
of the fluids being handled do not exceed 300 degrees F (see Figure 2).
Take the time to meet with your
state and municipal elected officials and don't ignore the town engineer.
The more you know about local capabilities for handling liquid plant wastes,
the better.
Nonmetallics don't corrode. When identified as being compatible with a
given material or group of materials, plastics are chemically inert.
Abrasion resistance is also critical, particularly when handling waste
streams with solid particles and debris. Here, too, the nonmetallics are
superior to metals. Note the wide difference in weight loss by abrasion
of the different materials shown in
Figure 2. Abrasion resistance directly impacts on pump performance
and dependability.
The single most common nonmetallic material recommended for pumps handling
waste streams is polypropylene. This thermoplastic is chemically inert
to most acids, alkalis, and solvents. It has become a standard for waste
treatment and neutralizing because of its broad compatibility with diverse
dilute chemicals.
Polyvinylchloride is also in wide use for handling acids and alkalis,
but not for solvents. Because of its good physical properties, it has become
an industry standard pipe. For the handling of strong oxidizing acids,
chlorinated hydrocarbons and extremely corrosive abrasive materials, the
fluoropolymers, particularly polyvinylidene fluoride, are recommended.
Fiberglass reinforced plastics are also used where the corrosive nature
of the fluid permits. Because of their composite structure that uses glass
fiber strands in a resin matrix, fiberglass reinforced plastic is a poor
pump choice for handling abrasive liquids or slurries.
When in doubt, the safe move is
to err on the side of caution. Let experience guide you as well as the
corrosion tables.
Most tanks are safely specified in polypropylene, polyvinylchloride, PVDF,
or fiberglass reinforced plastics. Metal tanks with nonmetallic linings
are suggested when pressure ratings are higher than those permissible for
thermoplastic or thermoset tanks. Material selection should always take
into consideration your current applications as well as a look into the
future. When in doubt, the safe move is to err on the side of caution.
Let experience guide you as well as the corrosion tables.
The law
Regulations are written by lawyers and often unintelligible to mortals.
What follows is a summary of the pertinent regulations relative to the
discharge of liquid wastes. It has been prepared from data received from
Jeffrey Kozel, a chemical/project engineer with Dynamac Corporation, a
major environmental consulting firm whose origin coincides with the creation
of the EPA in 1970. The old adage, "you can catch more flies with honey
than with vinegar" is a good one to remember.
Confrontation--whether you win, lose, or draw--is seldom profitable
to anyone but the lawyers. On the good news side is the fact that the government
is working hard on improving its image. Discussions we have had recently
with OSHA and EPA officials show a strong trend toward having their operations
prove user-friendly.
Clean Water Act. Its overall purpose is to assure that the nation's
waters are safe to the public and support fish and other stream life. Over
the years it has been clarified and amended with regulatory and enforcement
rulings calling for compliance and requiring specific discharge permits.
Funds were made available by the federal government to assist municipalities
in the construction of sewage treatment plants to help them meet mandated
requirements.
These publicly owned treatment works were required to be in compliance
within preset time periods. Since the federal monies were given to the
states to enhance their role in the management and control of construction,
and since the states were authorized to establish water quality standards
with respect to effluent limits and compliance schedules, it is obvious
that there is room for negotiation.
National Pollutant Discharge Elimination System. This program,
established under Clean Water Act, refers to the discharge permits issued
by EPA and the approved states. These permits are required by any source
directly discharging pollutants into navigable waters. More than 60,000
permits have been issued. Indirect discharges through publicly owned treatment
works are regulated under a separate program. These permits are specific
with respect to average monthly and maximum daily levels, concentration
and compliance schedules. Specific monitoring, testing, and reporting requirements
are included. The statute was subsequently modified to include the introduction
of toxic and hazardous substances into surface waters. The values were
to be set in accordance with best management practices established
and enforced on a case-by-case basis. The intent is to reduce secondary
pollution such as those caused by raw material storage piles which require
coverage against rain, and protection against run off.
Effluent guidelines. EPA was authorized to set restrictions on
pollutants discharged at industrial plant outfalls. These were usually
set by weight per fluid volume or by the chemical aggressiveness of the
fluid expressed by its pH value.
There are three levels of technology affecting existing industrial sources.
Technology (BPCT),
-
Best Conventional Technology (BCT), and
-
Best Available Technology Economically Achievable (BAT)
Although arbitrary compliance due dates were originally set and extended,
and all set dates have passed, the basic concepts are still in effect.
Because of understandable difficulties with existing industrial sources,
the specific requirements and compliance dates were permitted to be negotiated
and adjusted, but they couldn't be ignored.
Confrontation--whether you win, lose, or draw--is
seldom profitable to anyone but the lawyers.
Industrial sources of pollution are now governed by New Performance Source
Performance Standards. These are different for each industrial category
and must be achieved when discharge begins. The application for a permit
must include detailed information concerning every aspect of plant operation,
plus analytical testing and monitoring for every conceivable pollutant,
including pH of the discharge fluid.
 Reporting
requirements. The regulations require that discharge monitoring reports
be submitted on a regular schedule. Noncompliance reports must be submitted
describing the reason for the noncompliance, the expected date for return
to compliance, and plans to minimize or eliminate the recurrence. If the
discharge involves toxic pollutants, threat to drinking water or injury
to human health, EPA must be notified within 24 hours of the event. Reporting
requirements. The regulations require that discharge monitoring reports
be submitted on a regular schedule. Noncompliance reports must be submitted
describing the reason for the noncompliance, the expected date for return
to compliance, and plans to minimize or eliminate the recurrence. If the
discharge involves toxic pollutants, threat to drinking water or injury
to human health, EPA must be notified within 24 hours of the event.
Pretreatment standards for
indirect discharges to publicly owned treatment works. The setting
of these pretreatment standards is related to the capability of the publicly
owned treatment works to accept and treat the effluent from individual
industrial sources. They have full authority to regulate and control any
plant effluent that might adversely affect their sewage treatment and their
own compliance.
Corrosivity of the effluent is specifically listed because of its impact
on piping or control instrumentation, as well as on the deleterious effect
of corrosive chemicals on biological activity and human health.
Municipal and industrial stormwater permits. EPA established
a stormwater permit program. The permits issued require municipalities
to reduce pollutants to the maximum extent practicable for municipalities
or to technology-based requirements for industry. Immediate corrective
action must be taken when a discharge contributes to a violation of a water
quality standard or is a significant contributor of pollutants to the nation's
waters.
Copyright May 1998 Plant Services on the WEB
|