Solving VOC and odor problems
Biofiltration systems take on air pollution
challenges around the world
Michael S. McGrath, Product Manager, Monsanto Enviro-Chem Systems,
St Louis, Missouri
Biological oxidation and biofiltration technology are earning a winning
track record quickly when it comes to air pollution control. How? It's
simple. Biofilters operate at low ambient temperatures and do not require
chemicals or supplemental fuels. This environmentally-friendly technology
is cost-effective to operate, easy to maintain, and safe to use in populated
areas.
Biological oxidation is proving its success in handling low concentrations
of volatile organic compounds and nuisance odors at installations involving
industries from food flavoring and fragrance to municipal and industrial
wastewater treatment. Further, many large, well known industrial companies
around the world chose biofiltration to solve their pollution discharge
problems.
 The
process The
process
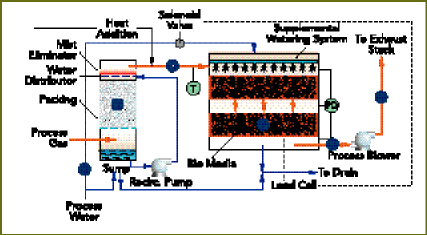
Biofiltration see (Figure 1) eliminates unwanted chemical components
from offgassing by means of biological oxidation. The biofiltration system
ductwork conveys contaminated gases containing volatile organic compounds
or odors to a humidification vessel. The system forces the gas to flow
upward through plastic packing and falling water. This countercurrent operation
saturates the contaminated air with water vapor.
Figure 1
Once saturated, contaminated air enters an upper plenum of the biological
oxidation vessel and passes downward through a biologically active media.
As this occurs, contaminante in the gas stream diffuse into a water film
surrounding the biologically active media. Microorganisms in the water
film oxidize the contaminants and use the energy released for maintenance
of their own cell material and growth.
During biological oxidation, organic compounds consisting of carbon,
hydrogen, and oxygen degrade fully into carbon dioxide (CO2)
and water. Inorganic components, such as ammonia (NH3) and hydrogen
sulfide yield compounds that form acids. In some cases, these compounds
must be neutralized.
The purified gas collects in the bottom of the biological oxidation
vessel before exiting. The cleaned air then enters a process blower and
discharges to the atmosphere through a stack.
Biological filter material
The performance of any biofilter is largely dependent on high quality
filter media. An effective biofilter media has specific characteristics.
The filter media should provide nutrients for cell growth and a high specific
surface area for attached microorganisms. Also, the media should have a
low gas pressure drop, promote uniform gas distribution, compaction, have
proper pH, long term operation, and optimal water content.
Microorganisms
Microorganisms are the heart of a biofiltration system. Every aspect
of the system should provide optimal conditions for the microorganisms.
They need, among other things, carbon and energy sources to synthesize
new cell material and growth.
Two groups of aerobic microorganisms--those that require oxygen for
life--are important to microbial conversion of contaminants in the filter
material. The first is autolithotrophic and the second is heteroorganotrophic.
Autolithotrophic microorganisms use carbon--present in the off gas stream
as carbon dioxide--to produce new cell material. Autolithotrophic bacteria
obtain their energy by converting inorganic compounds.
The majority of microorganisms used for biofiltration are heteroorganotrophic.
Heteroorganotrophic bacteria use carbon in the gaseous pollutant as sources
for growth and other cell functions. Most organic compounds can be used
by heteroorganotrophes. Only the rate at which the bacteria use compounds,
differs.
Heteroorganotrophes in biofilters vary somewhat in nature. Some species
are not capable of synthesizing the necessary compounds for growth from
one, or a few, carbon sources. Additionally, microorganisms require vitamins
and certain amino acids for cell production.
Both types are chemotrophic. That means that the required energy for
cell function is obtained from chemical reactions. This is in contrast
with phototrophic bacteria that use sunlight as an energy source.
There are only a few inorganic compounds in nature suitable for this
type of degradation. Of these, only ammonia and hydrogen sulfide are important
to biofiltration.
The gaseous compounds are converted into non-gaseous salts. The salts
accumulate in the filter material and, in some cases, must be rinsed from
the system periodically.
Nitrosomonas and nitrobacter are the autolithotrophic
organisms that convert ammonia and nitrate to nitrate and nitrite, respectively.
Thiobacillus is mainly responsible for converting hydrogen sulfide to sulfate.
The most effective biofilter media
consists of a mixture of specialized compost, pre-inoculated bacteria,
inert spherical material, and limestone.
Media
The most effective biofilter media is a mixture of specialized compost
pre-inoculated with bacteria, inert spherical material, and limestone.
The ratio of these components is crucial and should be optimized through
rigorous investigation.
Specialized compost provides the necessary nutrients for the bacteria.
Because of its physical structure, the compost minimizes gas pressure drop.
Inert spherical material provides for a low gas pressure drop, uniform
gas flow, and resists compaction over time.
The filter material should be preinoculated with specially chosen bacteria
during manufacture and mixing. This ensures optimal performance and dramatically
reduces acclimation periods compared with non-inoculated media. A calculated
amount of limestone in the media provides buffering for each particular
application.
Microorganisms in the water
film oxidize the contaminants and use the energy released for maintenance
of their own cell material and growth.
Moisture content
Complete removal of a target compound depends on the proper operation
of the biofiltration media. The compound must be absorbed into a liquid
biofilm surrounding solid media particles. For this reason, the biofilter's
moisture content is extremely important.
If the biofilm is improperly formed, the absorption step will not be
optimized and the biofilter's performance suffers. For the biodegradation
step to be most effective, the liquid film must have the proper thickness.
If the biofilm is not thick enough or absent in some part of the media,
the ultimate density of microorganisms can not be reached. If this happens,
the biofilter performs below expected levels.
On the other hand, if the biofilm is too thick, oxygen does not penetrate
the inner portions of the film. If this occurs, anaerobic growth forms
in the inner portions of the biofilm and reduces the effectiveness of the
system. In addition, excessive water plugs open pores in the media.
Anaerobic growth produces hydrogen sulfide as an extremely odorous byproduct.
Anaerobic conditions and density restrictions can be eliminated if the
proper moisture content is present in the media.
To ensure proper moisture content, the biofilter media must be weighed
continuously. This is possible when the system incorporates support beams
resting on load cells to sense the weight of the media. A programmable
logic controller continuously calculates the media moisture content based
on the filter material's weight. If the weight indicates the bed is below
the optimal moisture content, the controller activates spray nozzles above
the media bed. These water sprays add water to the media based on a predetermined
duration relative to the dryness of the media.
However, this type of a moisture control system only works on a fully
enclosed biofilter system. Open top type biofilters are always susceptible
to periods of excessive drying and wetness of the media.
Nutrients
Diffusion from the compost particles and from dead biomass supply nutrients
to the biofilm. For this reason, compost selection is critical to the success
of a biofilter. The most efficient compost is high in nitrogen content
and contains acceptable levels of phosphorus. Other types of nutrients
are also necessary, but are only required in trace levels.
System sizing
The following information addresses the factors listed in Table 2 and
explains how each factor affects system size--
the volume of media required for a particular application.
Table 2
FACTORS AFFECTING SYSTEM SIZING
|
Factor
|
Affect on system size
|
| Increased solubility |
Decrease |
| Reduced solubility |
Increase |
| Increased biodegradability |
Decrease |
| Reduced biodegradability |
Increase |
| Low removal efficiency required |
Decrease |
| High removal efficiency required |
Increase |
| Single compound to remove |
Decrease |
| Multiple compounds to remove |
Increase |
| Acid forming compounds H2S present
with VOCs |
Increase |
Solubility
Unlike other volatile organic compound and odor abatement technologies,
biofilter function and sizing depends on many variables. For example, compounds
treated in a biofilter must be water soluble. If a compound is easily transferred
from the gas to the liquid phase within a biofilter, the biofilter is sized
appropriately small.
However, when trying to remove slowly transferred compounds like aromatics,
then the biofilter can become quite large.
As with a packed column, mass transfer into the liquid phase is controlled
by a biofilter design. Consequently, water solubility of a compound largely
dictates system size.
Biodegradability
Water solubility is not the only variable dictating system size. Once
the compound is transferred into the liquid phase within the biofilter,
the microorganisms present must oxidize the compound. The speed of this
biological step depends on the compound being oxidized or biodegraded.
Molecular weight is usually an indicator of biodegradability. The higher
the molecular weight, the more difficult the biodegradation. Also, aromatics
are more difficult to break down than straight chain compounds.
Removal efficiency required
In a biofilter, the required removal efficiency is a major factor in
determining system size. A biofilter designed to remove 20 ppm of toluene
at 95 percent efficiency must be much larger than a biofilter designed
for 80 percent removal. In the first case, the amount of contaminant mass
that must be removed is greater than the second case. Therefore, the biofilter
must have more media for additional mass transfer and biodegradation.
Number of compounds to be removed
The quantity of compounds requiring removal also affects system size.
Microorganisms typically degrade more desirable compounds --those easy
to break down and high energy yield--before degrading other compounds.
The required volume of media does not double or triple with the addition
of compounds but can increase system size significantly.
Hydrogen ion
Finally, the impact of hydrogen ion --pH--on biodegradation varies
for each compound. The most common occurrences of acidic media formation
involve the destruction of hydrogen sulfide that eventually produces sulfuric
acid and reduces the pH of the biofilter media, specifically the pH of
the biofilm.
Hydrogen sulfide degrading organisms survive at pH levels as low as
2 while volatile organic compound degrading organisms require a near neutral
pH. Therefore, if volatile organic compounds must be removed in the same
biofilter as hydrogen sulfide, provisions must be made to keep the biofilter
neutral.
To prevent acidification, limestone is added to the media in these types
of systems. The limestone reacts with the sulfate produced in the biofilter
and creates gypsum. Due to the formation of gypsum, only a limited quantity
of limestone can be added to a given volume of media. Therefore, the amount
of lime, required to maintain a neutral media pH, can dictate the size
of some systems.
Regulatory compliance
Many companies use biofiltration, not only for odors, but for volatile
organic compound control. Although odor emissions are sometimes regulated
in North America, most often volatile organic compound control is regulatory
driven. Regulatory agencies typically mandate that 95 percent or more of
volated organic compounds be removed.
Biofilters meet these demands economically when the compounds and inlet
concentrations are favorable to biofiltration. Namely, gas streams containing
highly water soluble compounds with inlet concentrations below 300 milligrams
per cubic meter make ideal biofilter applications.
Other technologies
Thermal oxidation or catalytic technology--When is a biofilter
more suitable than thermal oxidation or catalytic technology? Some simple
screening tools are useful when trying to decide if an application favors
thermal oxidation or biofiltration.
Thermal technologies are a better choice most of the time if the inlet
concentration of the air stream is in the autothermal range--no supplemental
fuel required for continuous operation. Typical autothermal concentrations
for catalytic oxidizers are between 500 to 1,000 milligrams per cubic meter
of contaminants. For non-catalytic systems, concentrations usually start
at around 1,000 milligrams per cubic meter If the required removal efficiency
is above 98 percent, thermal oxidation is the correct choice.
With biological systems, microorganisms
typically degrade more desirable compounds --those easy to break down and
high energy yield--before degrading other compounds.
The one exception to this rule is inlet concentrations below 200 milligrams
per cubic meter. Whenever, the inlet concentration is low, thermal technologies
require supplemental fuel and can become extremely expensive to operate.
In addition, biofilters are sized differently than thermal technologies.
Biofilters are based on the amount of mass and contaminant that must be
removed. The physical size of thermal oxidizers usually are not dependent
on loading or contaminant type. Therefore, if the inlet concentration is
low, the biofilter required to achieve the desired removal may be quite
small. Consequently, a biofilter incurs less capital and operating costs
than a thermal oxidizer to perform the same task.
Chemical scrubbers--When is a biofilter more suitable than a
chemical scrubber? Both packed bed scrubbers and biofilters require that
the compound to be removed be water soluble. The difference in the technologies
occurs once the contaminant has been transferred from the gas to the liquid
phase. Biofilters use microorganisms to oxidize compounds while chemical
scrubbers rely on chemical reactions.
When using chemical oxidation, chemicals must be supplied to the system
continuously. The continuous addition of chemical costs money while microbiological
reactions do not. However, the capital investment of a scrubber is usually
less than a biofilter for a given situation. Again, an exception to this
is low inlet concentrations.
Another difference is liquid discharge. A chemical scrubber has a significant
liquid effluent that can have a high or low pH. If this is a concern, use
a biofilter with minimal liquid discharge.
Activated carbon--When is a biofilter more suitable than activated
carbon? The expense associated with activated carbon is not the capital
investment but in the carbon replacement and regeneration costs. Carbon
systems are designed on the basis of flow and the regeneration cost is
set by the inlet load to the carbon. Also, when the carbon can no longer
be regenerated, it is disposed of as a chemical waste. Activated carbon
achieves high removals but tends to be expensive if the inlet load is high.
Biofilters are more expensive to install than carbon system but, if the
inlet load is moderate to high, the payback duration of a biofilter can
be quite short.
Summary
Although installed in many plants throughout Western Europe for over
15 years, industries in North America have embraced biofiltration technology
only recently. Consequently, successful, long-term applications are just
now gaining recognition. Potential users are realizing that biofiltration
is a low cost, safe and effective solution for volatile organic compound
and odor emission problems.
Copyright May 1998 Plant Services on the WEB
|